
Rapid and Accurate Determination of the Higher Order Structure of a Library of Proteins Using Microfluidic Modulation Spectroscopy
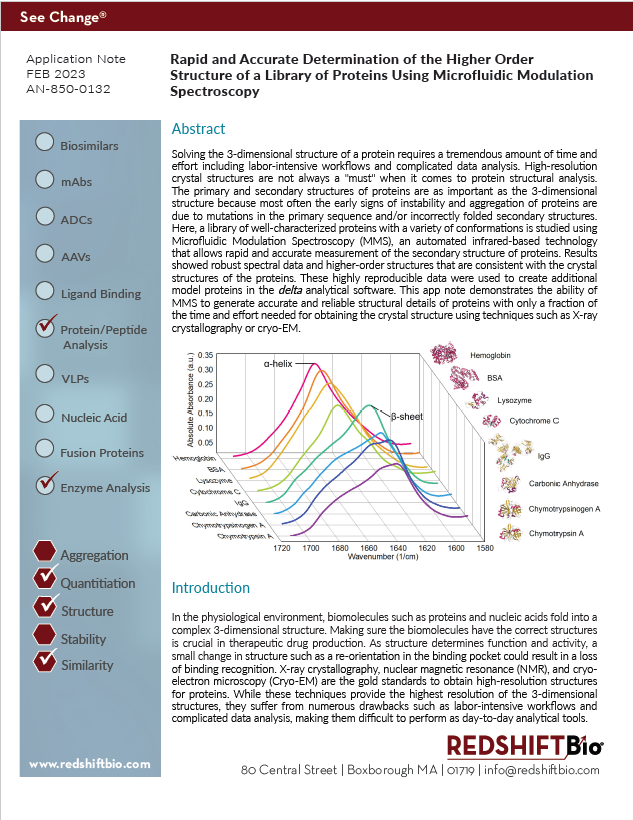
This app note studies the higher order structure (HOS) of eight different proteins with a variety of secondary structures: Hemoglobin, BSA, Lysozyme, Cytochrome C, IgG, Carbonic anhydrase, Chymotrypsinogen A, and Chymotrypsin A, and then compares the results to structural data obtained by FTIR, X-ray crystallography and AlphaFold.
Learn why MMS is a reliable method to obtain protein structural information that concurs with these other techniques while requiring only a fraction of the time and effort to generate. The repeatability of measurements for these different proteins at 10 mg/mL is consistently over 99.8%, and over 98% for samples at 1 mg/mL, providing an extreme level of confidence for formulation groups that need to generate high-quality data for their drug products at high concentrations. In addition, this data expands the protein database (protein library) in our MMS delta software for a wider variety of protein samples to choose from.

Please complete the form to download the app note.

