
Quantitation
Accurate Quantitation with Microfluidic Modulation Spectroscopy (MMS)
The ability to quantitate a sample and compare its measured concentration against a nominal value is a built-in benefit of the Aurora, powered by Microfluidic Modulation Spectroscopy (MMS). The Aurora TX is an unique IR spectroscopic tools that utilize a quantum cascade laser (QCL) and a microfluidic flow cell to provide secondary structural characterization of biomolecules. As opposed to using a chromophore, fluorophore or a fluorescent tag, IR spectroscopy probes absorption in the range of 780 nm to 1.0 mm resulting from the vibration of bonds present in a chemical structure. For proteins, the vibrations of interest result from bonds present in the peptide backbone, primarily carbonyl bending and stretching vibrations. Quantitation is automatically determined during routine sample analysis from the same differential absorbance spectra as the other spectral measurements. The improved sensitivity and real-time buffer referencing generated by MMS technology produces highly sensitive and linear results across a wide working concentration range of 0.1 to > 200 mg/mL.
How MMS Enables Precise Protein Quantitation
The value of concurrent quantitation among the other sample measurements using the Aurora lies in the ability to correlate concentration information with structural measurements as well as orthogonal measurements. This allows the scientist to assemble the information into actionable decisions and monitor the status of the biotherapeutic through the development and formulation processes.
Quantitation Example: Monoclonal Antibody Analysis
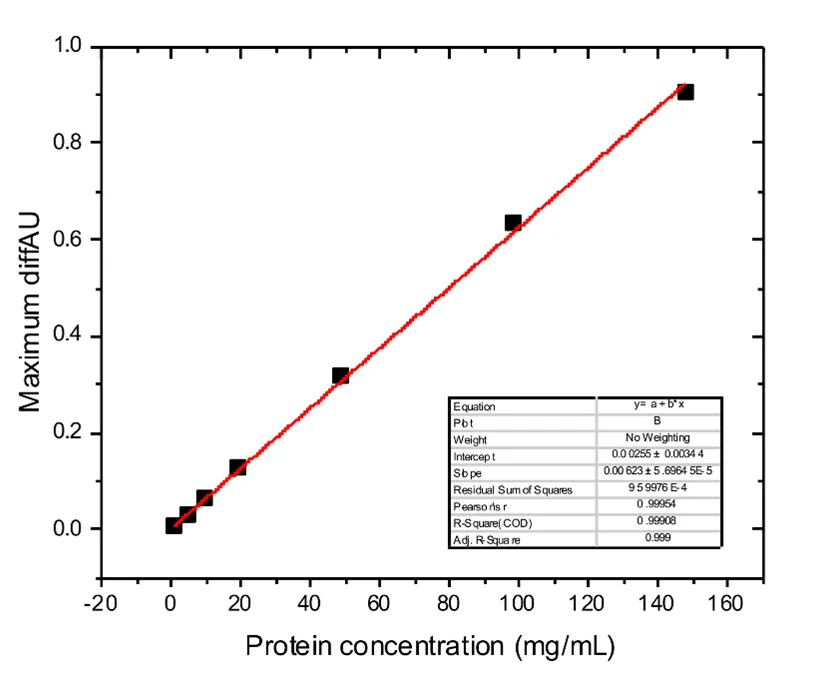
Quantitation results for a monoclonal antibody measured across the experimental concentration range of 1 to 150 mg/mL and linear with a correlation coefficient R2= 0.999. Application Note Monoclonal Antibody Analysis by Microfluidic Modulation Spectroscopy in a Complex Formulation Buffer (1 to 150 mg/mL).

